As of July 20, 2017, there have been at least 363 IPR petitions filed against patents that were listed in the FDA Orange Book, and 74 IPR petitions filed against patents that have been identified as reading on FDA Purple Book (CDER) listed biologic drugs. Of these 437 drug patent IPRs, 116 resulted in a final written decision ("FWD"). There are a number of lessons to be learned from these FWDs. We highlight here five of the most interesting.
Eighty-Five Percent of Drug Patent FWDs Concerned Patents That Have Also Been Litigated
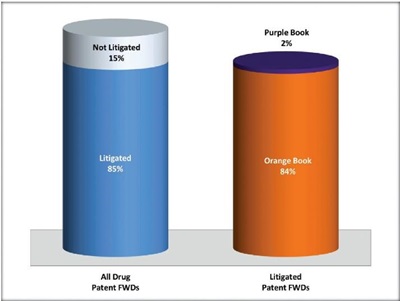
First, 85% of these 116 FWDs concerned drug patents that have also been challenged in Federal district court litigation. What is interesting, although perhaps not surprising, is that the overwhelming number of the FWDs on litigated drug patents concerned Orange Book listed patents. Of the 103 FWDs on Orange Book patents, 94% concerned patents that have been challenged in litigation. The numbers for CDER-listed biologic drug patents ("Biologic Drug Patents") are much lower: there were only 13 FWDs on Biologic Drug Patents, only 15% of which concerned patents that have also been challenged in litigation. These lower numbers for Biologic Drug Patents may not be surprising for a number of reasons: (1) far fewer Biologic Drug Patents have been challenged to date, both in litigation and in IPR; (2) there are far fewer FDA-approved biosimilar drugs than there are generic versions of Orange Book listed reference drugs; and (3) the Biologics Price Competition and Innovation Act of 2009 (BPCIA) "patent dance" (42 U.S.C. § 262(l)) provides a convenient procedure, in advance of market launch, to litigate a narrow subset of biologic drug patents, perhaps reducing the value of pre-emptive IPR challenges.
Patent Claims Have Been Found Unpatentable in More than Fifty Percent of Drug Patent FWDs
 Second, in the 116 drug patent FWDs we analyzed, the Patent Trial and Appeal Board ("PTAB") found all instituted claims unpatentable in 64 (55%) of these FWDs; some instituted claims unpatentable and some not unpatentable in two (2%) of these FWDs; and all instituted claims not unpatentable in the remaining 50 (43%) of these FWDs. In other words, if a drug patent IPR reaches a FWD, the odds are that at least some challenged claims will be found unpatentable. To succeed in IPR, a Petitioner must show that the challenged patent claims are unpatentable by a preponderance of the evidence. This is a lower standard than the clear and convincing standard used in litigation, and may, at least in part, explain the relatively high rate at which patents, across all technologies, are being found unpatentable in IPR. Other reasons include the broader claim construction standard (broadest reasonable interpretation) used in IPR, and the restricted ability to amend claims in IPR to avoid prior art.
Second, in the 116 drug patent FWDs we analyzed, the Patent Trial and Appeal Board ("PTAB") found all instituted claims unpatentable in 64 (55%) of these FWDs; some instituted claims unpatentable and some not unpatentable in two (2%) of these FWDs; and all instituted claims not unpatentable in the remaining 50 (43%) of these FWDs. In other words, if a drug patent IPR reaches a FWD, the odds are that at least some challenged claims will be found unpatentable. To succeed in IPR, a Petitioner must show that the challenged patent claims are unpatentable by a preponderance of the evidence. This is a lower standard than the clear and convincing standard used in litigation, and may, at least in part, explain the relatively high rate at which patents, across all technologies, are being found unpatentable in IPR. Other reasons include the broader claim construction standard (broadest reasonable interpretation) used in IPR, and the restricted ability to amend claims in IPR to avoid prior art.
Sixty-Two Percent of Drug Patent FWDs Have Been Duplicative FWDs With Identical Outcomes
Third, one of the most surprising findings when we analyzed these 116 drug patent FWDs was the high number—62%—that were duplicative FWDs (i.e., FWDs that issued on drug patents that were also the subject of at least one other FWD, whether or not through joinder). One of these duplicative sets of FWDs concerned a Biologic Drug Patent—a patent that was identified as reading on Humira®—the remaining 30 of these duplicative sets concerned various Orange Book listed patents. In all of these duplicative sets, either all instituted claims were found unpatentable, or all instituted claims were found not unpatentable. Further, all of these determinations were made on the ground of obviousness, and in each case, the Petitioner relied on at least one prior art patent or reference that had been cited during prosecution.1
A patent claim is deemed unpatentable as obvious, if the differences between the subject matter of the challenged claim and the prior art are such that the subject matter of the claim, as a whole, would have been obvious at the time the invention was made, to a person having ordinary skill in that art ("POSA"). Where an obviousness challenge rests on multiple prior art references, the challenger must show that there was a motivation or reason that would have prompted a POSA to combine the various teachings of these references in the way the claimed invention does. A challenger must also show that a POSA would have had a reasonable expectation of success when combining such elements. Obviousness is determined by looking at various factors, including: (1) the scope and content of the prior art; (2) the differences between the subject matter of the challenged patent claims and the prior art; (3) the level of skill in the art; and (4) objective evidence of non-obviousness, for example, unexpected results, long-felt unmet need, industry praise, and commercial success.
All instituted claims were found unpatentable in several sets of FWDs concerning patents that are Orange Book listed for at least the following drugs: Gattex®, Kerydin®, Thalomid®, Revlimid®, Pomalyst®; and the CDER Purple Book listed drug Humira®. All instituted claims were found not unpatentable in sets of FWDs concerning patents that are Orange Book listed for at least Gralise® and Prolensa®. The FWDs concerning Prolensa® are particularly interesting, because they highlight the importance and value of objective evidence in the non-obviousness analysis.
In March and April 2015, various InnoPharma and Mylan companies, and various Lupin companies filed two IPRs (IPR2015-00902 and -01099, respectively), challenging all 30 claims of U.S. Patent No. 8,669,290 ("the '290 Patent") as obvious, based on one prior art patent that was cited during prosecution, and one new prior art patent in each of the two IPRs. The '290 Patent concerns aqueous liquid preparations for ophthalmic administration, consisting of two components: bromfenac (or its salts or hydrates) (a non-steroidal anti-inflammatory drug (NSAID)), and tyloxapol (which stabilizes the bromfenac component). Oral hearings were held in April and June 2016 before the same panel of PTAB judges, and FWDs were issued in July and September 2016.
 In both IPRs, the PTAB found that the prior art patent listed on the face of the '290 Patent disclosed every limitation of claim 1, except for one limitation that was supplied by the newly cited prior art references: the use of a specific concentration of tyloxapol, instead of polysorbate 80. The PTAB found that based on the evidence in the record, a POSA would have known that polysorbate 80 and tyloxapol could be substituted successfully and predictably, because these compounds had previously been used interchangeably in ophthalmic formulations. The PTAB also found that this known interchangeability was sufficient to establish a prima facie case of obviousness, even in the absence of an express suggestion to use tyloxapol. Applying (in IPR2015-00902) the recent Federal Circuit decision in WBIP, LLC v. Kohler Co., 829 F.3d 1317, 1328 (Fed. Cir. 2016)—which rejected the proposition "that objective considerations of non-obviousness can never overcome a strong prima facie case of obviousness"—the PTAB then considered the Patent Owner's evidence of unexpected results, commercial success and industry acclaim. The PTAB found that: (1) substituting polysorbate 80 for tyloxapol had the surprising and unexpected result of a significant improvement in the stability of bromfenac; (2) the claimed inventions were embodied in Prolensa®, and Prolensa® was commercially successful, at least in part, because tyloxapol lowered the pH to close to that of natural tears, so Prolensa® lacked the burning and stinging side effects of other treatments; and (3) Prolensa® had received significant industry acclaim based on benefits flowing from the use of tyloxapol. In short, on the preponderance of the evidence, this objective evidence tipped the balance in the Patent Owner's favor.
In both IPRs, the PTAB found that the prior art patent listed on the face of the '290 Patent disclosed every limitation of claim 1, except for one limitation that was supplied by the newly cited prior art references: the use of a specific concentration of tyloxapol, instead of polysorbate 80. The PTAB found that based on the evidence in the record, a POSA would have known that polysorbate 80 and tyloxapol could be substituted successfully and predictably, because these compounds had previously been used interchangeably in ophthalmic formulations. The PTAB also found that this known interchangeability was sufficient to establish a prima facie case of obviousness, even in the absence of an express suggestion to use tyloxapol. Applying (in IPR2015-00902) the recent Federal Circuit decision in WBIP, LLC v. Kohler Co., 829 F.3d 1317, 1328 (Fed. Cir. 2016)—which rejected the proposition "that objective considerations of non-obviousness can never overcome a strong prima facie case of obviousness"—the PTAB then considered the Patent Owner's evidence of unexpected results, commercial success and industry acclaim. The PTAB found that: (1) substituting polysorbate 80 for tyloxapol had the surprising and unexpected result of a significant improvement in the stability of bromfenac; (2) the claimed inventions were embodied in Prolensa®, and Prolensa® was commercially successful, at least in part, because tyloxapol lowered the pH to close to that of natural tears, so Prolensa® lacked the burning and stinging side effects of other treatments; and (3) Prolensa® had received significant industry acclaim based on benefits flowing from the use of tyloxapol. In short, on the preponderance of the evidence, this objective evidence tipped the balance in the Patent Owner's favor.
Drug Patents Challenged in Duplicative FWDs Are More Likely to be Found Unpatentable
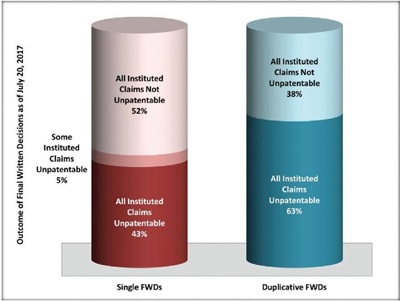
 Fourth, comparing the outcome when a drug patent was challenged in only one FWD, to the outcome when a drug patent was challenged in two or more FWDs, based on our dataset (116 FWDs), claims of drug patents facing multiple challenges were much more likely to be found unpatentable. Combining all duplicative FWDs on drug patents as of July 20, 2017—joined and not joined—the PTAB found all instituted claims unpatentable in 45 (63%) of these FWDs; and all instituted claims not unpatentable in 27 (38%) of these FWDs. The equivalent numbers for drug patents challenged in only one FWD were 19 (43%) (all instituted claims unpatentable); two (5%) (some instituted claims patentable, some unpatentable); and 23 (52%) (all instituted claims not unpatentable). In other words, this shows that challenged claims of drug patents are much more likely to be found unpatentable when there have been multiple FWDs on the same patent: 63% to 43%. This 20% difference seems significant, although it may be a product of the strength of the challenged patents themselves, rather than a "second bite of the apple" being more successful.
Fourth, comparing the outcome when a drug patent was challenged in only one FWD, to the outcome when a drug patent was challenged in two or more FWDs, based on our dataset (116 FWDs), claims of drug patents facing multiple challenges were much more likely to be found unpatentable. Combining all duplicative FWDs on drug patents as of July 20, 2017—joined and not joined—the PTAB found all instituted claims unpatentable in 45 (63%) of these FWDs; and all instituted claims not unpatentable in 27 (38%) of these FWDs. The equivalent numbers for drug patents challenged in only one FWD were 19 (43%) (all instituted claims unpatentable); two (5%) (some instituted claims patentable, some unpatentable); and 23 (52%) (all instituted claims not unpatentable). In other words, this shows that challenged claims of drug patents are much more likely to be found unpatentable when there have been multiple FWDs on the same patent: 63% to 43%. This 20% difference seems significant, although it may be a product of the strength of the challenged patents themselves, rather than a "second bite of the apple" being more successful.
Challenged Claims in Biologic Drug Patents Are More Likely to be Found Unpatentable
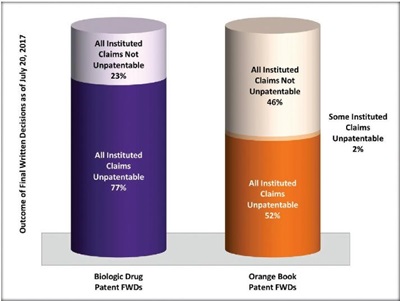
Finally, focusing specifically on FWDs concerning Biologic Drug Patents—of which there were at least 13 as of July 20, 2017—the PTAB found all claims unpatentable in 10 (77%), and all claims not unpatentable in the remaining three (23%) of these Biologic Drug Patent FWDs. While this is a small dataset, the outcome of these Biologic Drug Patent FWDs is markedly different from that for patents listed in the Orange Book. The comparative numbers for Orange Book patent FWDs were: all instituted claims were found unpatentable in 54 (52% of) FWDs; at least some instituted claims were found unpatentable in two (2% of) FWDs; and all instituted claims were found not unpatentable in 47 (46% of) FWDs. In short, if an IPR challenge to a Biologic Drug Patent reaches a FWD, the odds are significantly higher that all challenged claims will be found unpatentable. It will be interesting to see if this trend continues or changes as the Biologic Drug Patent FWD dataset becomes larger.
In sum, the biggest takeaways from the 116 drug patent FWDs we studied were that, first, the vast majority of these FWDs concerned patents that had also been litigated; and also concerned patents that had been challenged in at least one other IPR. Second, if an IPR challenge to a drug patent reaches a FWD, it is more likely than not that at least some instituted claims will be found unpatentable—and this risk may be higher for Biologic Drug Patents based upon the current limited dataset (n=13). Finally, robust objective evidence remains a very important tool in the arsenal to overcome obviousness challenges.
[1] In IPR2014-00379, the Petitioner, in addition, argued anticipation, but that argument was unsuccessful.